imec’s research work to advance biosensors
 Monday, July 19, 2021 at 5:15PM
Monday, July 19, 2021 at 5:15PM Part 3: Biosensor developments
- Pol Van Dorpe discusses the institute’s use of photonics and silicon to develop new designs for medical diagnostics.
- imec has designed a breathalyser that detects the coronavirus with the accuracy of a polymerase chain reaction (PCR) test, a claimed world first.
 Pol Van Dorpe, an imec Fellow
Pol Van Dorpe, an imec Fellow
Optics and photonics are advancing medical diagnostics in two notable ways.
The technologies are helping to shrink diagnostic systems to create new types of medical devices.
"Going from big lab equipment to something much smaller is a clear trend," says Pol Van Dorpe, a Fellow at imec, the Belgium R&D nanoelectronics and nanotechnology institute.
Photonics and silicon also benefit central labs by creating more powerful test instruments. More functionality and detectors can be integrated in a given area enabling multiple tests in parallel, a technique dubbed multiplexing.
imec’s biosensor work and espertise
imec began its biosensor research in the 1990s, investigating electrical and surface plasmon-based devices. In more recent years, it has added the development of custom biosensor chips for companies.
As examples, imec worked with Panasonic to develop a chip for PCR, a testing technique now known to the public due to covid-19. The R&D institute also worked with Genalyte, a pioneering silicon photonics medical diagnostics company that uses optical waveguides, ring resonators, and a tunable laser for its multiplexing biosensor product.
imec has also developed in-house expertise across several disciplines needed for biosensor development.
Several groups at imec cover photonics, with Van Dorpe heading the group addressing biophotonics and single-molecule electrical devices.
Another group addresses biochemistry and surface chemistry used to coat and activate a sensor’s surface so that receptors can be attached. Receptors are biochemical materials that enable the sensor to trap and detect materials.
A further team covers microfluidics used to deliver liquid samples to the sensor or to mix solutions precisely.
Semiconductor process steps are used to create high-aspect-ratio structures that implement microfluidic structures. Such structures can also be used to sort cells, known as cytometry.
“There are many sensor types, and each has its own fluidic needs,” says Van Dorpe.
Spin-offs
imec has also spun off several biosensor companies.
One, miDiagnostics, raised $16.5 million in funding in 2020. miDiagnostics has a nanofluidic processor (nFP) that performs diagnostic tests on fluids guided through the system using capillary forces. The liquids can be redirected and can even have their flow reversed.
The nFP is configurable depending on the application. It combines nanofluidic processing and PCR for processing biomarkers: from cells and proteins to nucleic acids and small molecules.
Indigo is another spin-off that is developing a glucose monitoring system. A photonics sensor is embedded under the skin and communicates the user’s blood sugar level to a smartphone.
Market trends
The biosensor market is complex. Many actors - labs, doctors and users - in healthcare must be convinced before adopting a biosensor device. For a device to be successful, it must add value compared to existing equipment. Cost is also key as is the use-case and ease of use.
Portable equipment that delivers results promptly so that medical staff can make quick decisions is one example. Others include identifying if a patient has suffered a heart attack or bacterial infections such as sepsis, or enabling a doctor’s office to determine if a patient has a bacterial or viral infection. But no doctor will have 20 devices in their office, each performing a specific test, he says.
Such biosensor devices could also have played a key role during the current coronavirus pandemic.
“I can tell you we were working with companies and if they were several years ahead in their roadmaps, much of this would have been a lot easier,” says Van Dorpe.
Antigen-based quick tests for covid exist but people don’t trust them completely due to their limited sensitivity. It is also still not known when people become contagious. “If you take a nasal swab but are already recovering then you may not be as contagious,” says Van Dorpe.
imec has developed a coronavirus breathalyser. Blowing into a filter, aerosols and small droplets from a person’s lungs are collected. A 5-minute PCR analysis unit delivers a result, informing the person if their breath is infectious.
The goal is to use such systems at airports and large events, but it doesn’t guarantee that a person won’t get sick. “You could have been infected the previous day,” says Van Dorpe.
In clinical trials with UZ Leuven, the university hospital of Leuven, the system has tested viral RNA in exhaled air with high sensitivity.
“Our chip technology can detect quickly the virus particles with the same accuracy as classical PCR tests,” says Van Dorpe. “We are the first in the world to demonstrate this.”
imec is undertaking more clinical trials while improving the test’s robustness and ease of use. “We are discussing the commercialisation of our technology with different parties,” he says.
Biosensor technologies
imec’s toolbox of technologies include silicon nitride optical waveguides, beam splitters, filters, spectrometers, and in-plane and out-of-plane lenses.
imec can deposit waveguides on CMOS and has exploited the technique with CMOS image sensors that have many detectors. “We can use commercial image-sensor wafers and deposit the waveguide technology and use those pixels as detectors,” says Van Dorpe.
Established diagnostic techniques used in medical labs include ELISA, a reference technique to detect proteins, and PCR that tests for nucleic acid detection (DNA/ RNA).
The importance of both lab techniques will not change anytime soon, says Van Dorpe.
One reason why ELISA and PCR are so established is that they use ‘amplification’ to detect minute amounts of the material being tested for - the analyte - in complex samples.
For amplification, another label is used in addition to the receptors. The analyte is attached to an antibody within the label, and it is this second label that provides greater testing sensitivity. This, however, requires sample preparation by trained staff.
In contrast, newer biosensors technologies such as surface plasmon resonance (SPR) and silicon photonics use a label-free approach that does away with the second analyte-label stage.
But the label-free sensor is less sensitive; the technique measures when something attaches to the receptors but it can’t distinguish what it measures.
Van Dorpe stresses that amplification is chemistry-related and so it can be used with silicon photonics or SPR.
It is the overall diagnostic system that determines sensitivity, the combination of the transduction process and the chemistry, says Van Dorpe.
SPR and silicon photonics
SPR and silicon photonics biosensors work by measuring changes in light caused by passing a sample over the sensor which causes molecules to attach to the surface.
An SPR system comprises a laser, a prism attached to a gold surface, and a detector. Light is shone through the prism and is reflected from the gold layer before being detected. At a certain incident angle, the light causes electron resonance on the gold surface causing the reflected light intensity to dip.
Attaching biochemical receptors to the gold surface tailored to the analyte causes a shift in resonance angle and the angle change is a measure of the analyte’s presence.
In contrast, silicon photonic designs measure refractive index changes in the light caused by analytes attached to receptors on the sensor’s surface. Two sensor designs are used: a laser with either a Mach-Zehnder interferometer (MZI) or a ring resonator.
“Everything that changes the refractive index causes a signal,” says Van Dorpe.
imec’s biosensor developments
imec’s work with Genalyte a decade ago involved a biosensor that used a tunable laser and ring-resonator sensors.
More recently, the R&D institute has developed technologies not reflected in the silicon photonics designs used by biosensor start-ups such as Genalyte, SiDx, Antelope DX and SiPhox.
imec’s biosensor technologies have been developed to be less sensitive to non-specific binding. What is measured is fluorescence that occurs with the binding to the analyte.
“In blood or more complicated samples, there is a lot of stuff [besides what is being tested for],” says Van Dorpe.
One technology imec has developed performs rapid ELISA-style testing without needing the repeated wash stages required with ELISA systems.
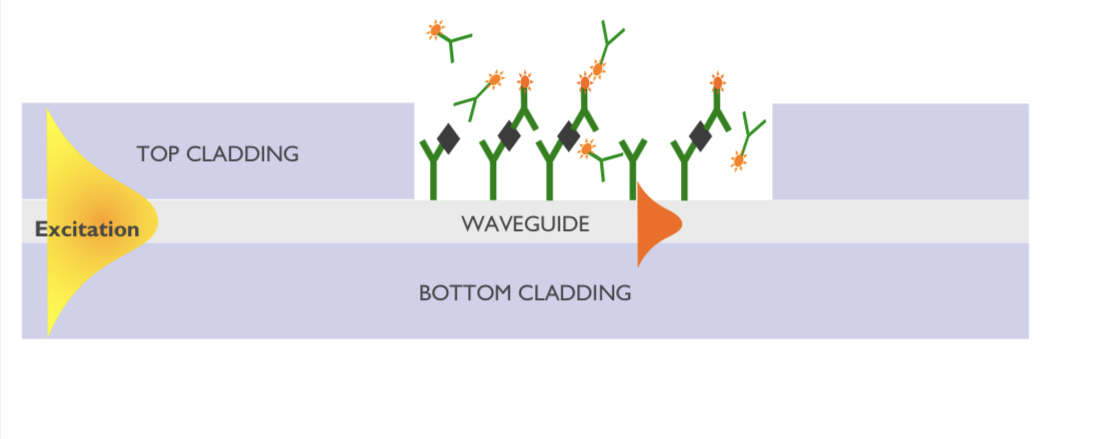 imec's waveguide flourescence-based sensor
imec's waveguide flourescence-based sensor
ELISA uses an antibody receptor to detect the tested-for material while a second antibody uses an enzyme that produces colour. And it is the colour that is measured. In effect, both antibodies detect the analyte but the second, with its fluorescent label, helps determine how much analyte has bound.
With standard ELISA testing, repeated wash steps are required to remove what has not bound to the receptors and labels. These wash stages lengthen the testing time.
imec’s sensor is sensitive in the region very close to the surface. Measuring the fluorescence near the surface determines its build-up over time (see diagram).
The cleverness of the sensor is that the larger the concentration, the faster the surface fills up, reflected in the rate of change of fluorescence over time.
“You don’t need to wait until everything has stabilised to determine the concentration,” says Van Dorpe. “You can wait, say 2 minutes, measure the slope of the signal and that gives you a direct measure of the concentration.”
The design can be used with blood samples, to measure protein production or proteins that shouldn’t be there.
The sensor allows the real-time measurement of biomarkers, and no wash stages are needed. It also enables a controlled process for the biological production of vaccines or cancer therapy.
The key here is that using waveguides and integrated photonics allows localised sensing.
“Also with waveguide technology, because you route light on a chip, you can address a lot of [sensing] sites at the same time,” says Van Dorpe. “That allows you to measure a lot of spots, what is called multiplexing.”
These are the advantages of integrated photonics: the ability to test in parallel and the precise quantification of concentrations, he says.
imec has developed a second fluorescence technique - called super-critical angled fluorescence - closely related to the first but that does away with the waveguide.
As with the first technique, two antibodies are used, one with a fluorescent label.
By exciting the fluorescent label, light is produced in all directions. If a high-angle beam is used, the light at the surface interface refracts within a critical angle.
A fluorescent molecule close to the surface - less than a wavelength away - emits light into a silicon-oxide material. This helps distinguish molecules far from the surface compared to closer ones.
imec’s compact system filters out fluorescence from labels floating further away while measuring those nearby. This is like what happens with the waveguide of the first technique, where it is routed to the detector. But here the detector is situated underneath to measure the fluorescence. The technique delivers rapid results.
The two imec techniques deliver selective sensing that doesn’t rely on refractive index changes or mass. With the latter techniques, all the signals are picked up: everything that binds, wanted and unwanted materials.
The imec techniques are not perfect. There is some degree of auto-fluorescence but it is low. Also, some antibodies with the label will bind to the surface but that is much smaller than the proteins, says Van Dorpe.
Cytometry
imec is working with Sarcura, a cell therapy firm, on a high-throughput cytometry solution for cell separation. Here photonic integration is used along with a microfluidic solution to measure the cells.
A standard cytometer has a flow of cells and a bank of lasers at multiple wavelengths typically. As the cells pass, they scatter the focused light from the lasers. The scattering is measured while the cells also fluoresce since they are labelled. This allows for cell categorisation.
With cell therapy for cancer treatment, immune cells are grown and need analysing. Another use is identifying tumour cells in the blood.
“There are lots of applications where you want to pick out specific cells, label them, look at their properties and classify,” he says.
Traditional equipment used for these tasks is large and complex, requiring skilled staff.
What silicon photonics and microfluidics allow is the bringing of cells to the channel and, with waveguides, illuminate them and detect them.
The result, says Van Dorpe, is a high-throughput design with many adjacent channels.
 COVID-19,
COVID-19,  ELISA,
ELISA,  PCR,
PCR,  Pol Van Dorpe,
Pol Van Dorpe,  SPR,
SPR,  biosensor,
biosensor,  imec,
imec,  microfluidics,
microfluidics,  multiplexing in
multiplexing in  Biosensors,
Biosensors,  silicon photonics
silicon photonics  Print Article
Print Article 


Reader Comments